Manufacturing Business

From a comprehensive perspective encompassing prevention, diagnosis, and treatment, we manufacture and market high-quality active pharmaceutical ingredients, pharmaceuticals, diagnostic reagents, and medical devices, that meet GMP* and QMS* requirements. We research, develop, manufacture and market unique and distinctive products, and undertake contract manufacturing of pharmaceuticals.
*GMP: Good Manufacturing Practice, standards for manufacturing control and quality control of pharmacenticals and quasi-drugs
*QMS: Quality Management System, standards for manufacturing control and quality control of medical devices and external diagnostic reagents
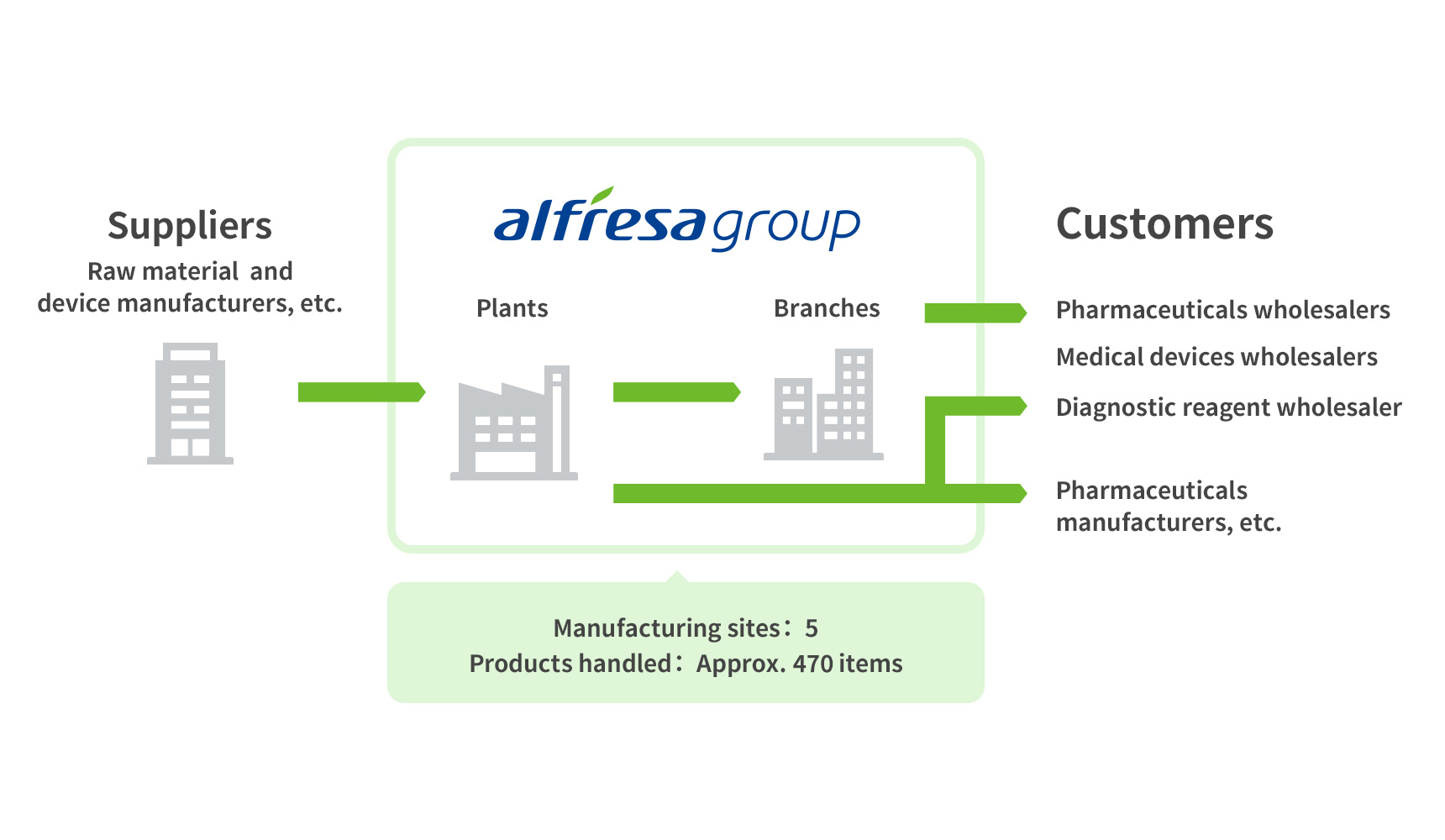
The figures shown are as of March, 2023.
3 Fields Handled by the Manufacturing Business
1Pharmaceuticals and Diagnostic Reagents
We market a variety of products focusing on ethical pharmaceuticals for neuropsychiatric and allergic disorders. We also deal in therapeutics for rare diseases and generic drugs. In the field of infectious diseases, we market diagnostic kits that aid rapid diagnosis in clinical and nursing settings. Through these products, we are contributing to early detection and treatment of diseases.
2Medical Devices
We are meeting increasingly sophisticated and diversifying needs in surgical settings. We offer a lineup of more than 10,000 varieties of surgical sutures, mainly for general surgery, cardiovascular surgery, and plastic surgery. We also market a cable system used as wiring materials in spinal fusion operations, surgical tools that use microwaves, and other devices.

3Active Pharmaceutical Ingredients and Contract Manufacturing
We manufacture and market high-quality active pharmaceutical ingredients that comply with both domestic GMP and U.S. CGMP standards. We also undertake contract manufacturing at the request of manufacturers in Japan and abroad under our sophisticated system of manufacturing and quality control.

Business Policy
Maximize corporate value by
"building a foundation for the next generation"
Reliable, Safe, and Sincere Manufacturing
- Promote sound manufacturing and sales of existing products while developing new products
- Reduce environmental impac
Efforts to Achieve Total Supply Chain Services
- Create a one-stop service that handles everything from development to manufacturing, logistics, and medication
- Introduce new manufacturing technology and production equipment that can respond to manufacturers' needs
New Initiatives That Utilize Digital Technology
- Innovate medical representative (MR) activities
- Venture into the field of digital therapeutics